3Aware Unveils Features for Label Expansion Support
August 05, 2025 - 3Aware, the leader in regulatory-grade real-world evidence (RWE) solutions for medical devices, today announced powerful features to support label expansion initiatives. These enhancements enable medical device manufacturers to identify off-label use, evaluate performance across patient subgroups, and generate the robust evidence required for new indications and market claims:
Cohort Stratification by Demographics & Procedure Codes
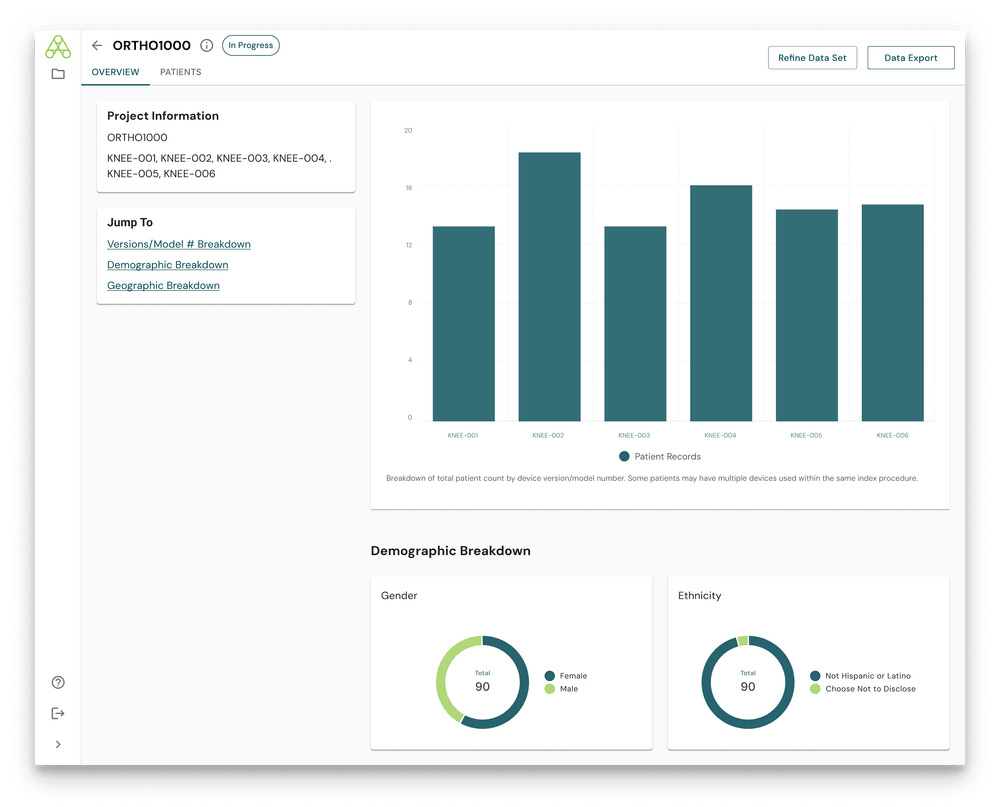
3Aware’s platform enables highly granular patient cohort creation using structured EHR data – including age, sex, race/ethnicity, diagnosis codes (ICD-10), and procedure codes (CPT, ICD-10-PCS). This allows users to:
- Identify precise subpopulations of interest (e.g., pediatric use, geriatric outcomes)
- Compare outcomes across labeled and potential off-label populations
- Track use trends by provider type, surgical setting, or region
Added Value for Manufacturers:
- Enables sponsors to evaluate product performance across subgroups
- Supports Clinical Evaluation Reports (CERs), Post-Market Surveillance (PMS), and targeted PMCF
- Helps manufacturers prepare evidence for label expansion submissions to regulators
Detection of Off-Label Use
By cross-referencing manufacturer labeling data with actual patient treatment patterns, the 3Aware platform can highlight:
- Clinical use cases that fall outside the device’s approved indication
- Real-world evidence of physician-led innovation or alternative patient applications
- Volume and outcome trends for off-label use over time
Added Value for Manufacturers:
- Offers manufacturers visibility into how their devices are being used outside the label
- Informs potential new indications supported by real-world data
- Surfaces safety and efficacy trends before formal label changes are initiated
Real-World Utilization Patterns by Indication and Patient Subgroup
The 3Aware platform allows for the surfacing of patterns in how devices are used across care settings and patient profiles, such as:
-
CPT code stratification (e.g., 27488 vs. 27590 for joint revisions)
-
Use in emergent vs. elective procedures
-
Adoption in high-risk or comorbid populations
Added Value for Manufacturers:
- Helps refine marketing strategy and product positioning
- Provides evidence for payer negotiations or clinical guideline inclusion
- Strengthens post-market clinical understanding of device performance across real-world populations
From Data to Decisions
3Aware’s platform gives manufacturers the ability to connect device-specific identifiers with comprehensive longitudinal patient data, including structured and unstructured EHRs, lab results, imaging, and PROMs. With this depth of evidence, manufacturers can confidently explore new indications, enhance regulatory strategies, and accelerate market access.
Book a demo with us to learn how these tools can support your next study!

